Sinopharm COVID-19 vaccine: All you need to know Dubai: The UAE, along with Bahrain, were two of the first Arab countries to approve the Sinopharm shot — and were the first countries to grant full approval to use a COVID-19 vaccine. Other countries followed suit. Here’s a closer look into the Sinopharm vaccine and how the UAE leads the world in the fight against the pandemic.
Who produces the Sinopharm vaccine?
Sinopharm’s China National Biotec Group (CNBG). Sinopharm is a Chinese state-owned biotech company based in Beijing.
What is the shot called?
BBIBP-CorV.
Where is it approved for use?
Reuters reported in September that Phase III trials for the Sinopharm shot had been conducted in 10 countries worldwide. The vaccine has been granted authorisation in China, and several other countries, including the UAE.
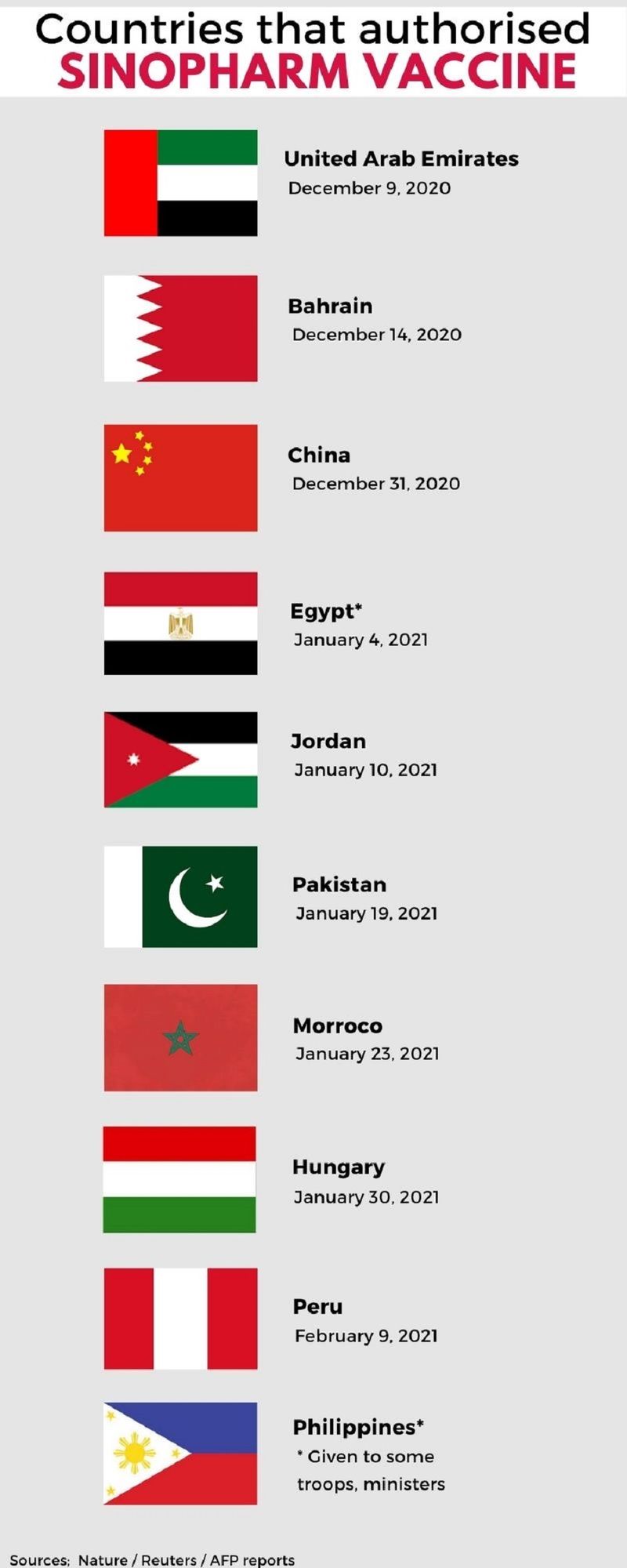
Why was the trial of Sinopharm, a Made-in-China vaccine, not done in China itself?
A vaccine trial is best done in places where a pathogen (like SARS-CoV-2) is still most active. In China, the case numbers had dropped significantly in the third quarter of 2020, due to the strict lockdowns. Doing a trial there would have made it difficult to obtain any meaningful data at all.
FREE OF CHARGE
• Four vaccines are approved in the UAE for use on eligible individuals against the COVID-19 infection: Sinopharm, Pfizer-BioNTech, Sputnik V and Oxford-AstraZeneca. These shots are offered to UAE citizens and residents — free of charge.
• As of February 20, 2021, the UAE has already recorded 56.15 doses of COVID-19 administered per 100 people, the second-highest in the world.
What method was used to develop the vaccine?
The vaccine is based on an “inactivated” virus.
How does the Sinopharm vaccine trigger an immune response?
Once inside the body, given through intra-muscular injection, some of the inactivated viruses are swallowed up by a type of immune cell called an “antigen-presenting cell”. The antibodies also attach to viral proteins, such as the so-called spike proteins that populate the viral surface.
4HUMANITY
Sinopharm vaccine is the same vaccine used in the groundbreaking 4Humanity’ trials, the first global clinical Phase III trial of an inactivated vaccine to combat COVID-19 (launched in UAE in July last year), and later was officially registered by the UAE Ministry of Health and Prevention (MOHAP) on December 9, 2020.
How is the Sinopharm vaccine made?
The journal Cell explains: to create Sinopharm’s BBIBP-CorV shot, the researchers obtained three variants of the coronavirus from patients in Chinese hospitals. They picked one of the variants because it was able to multiply quickly in monkey kidney cells grown in bioreactor tanks.
What’s the deal with inactivated virus vaccine?
It’s a tried-and-tested platform. Inactivated viruses have been used for over 100 years. Polio vaccine inventor Jonas Salk used them to create his polio vaccine in the 1950s. It’s the same method used to prepare vaccines against other diseases including rabies and hepatitis A. Coronaviruses in BBIBP-CorV are literally killed (inactivated) through a chemical process, so they can be injected into your arm without causing COVID-19.
How is the coronavirus used in the vaccine ‘killed’?
Yes, they are literally killed. But first, before they’re killed, lab workers produce huge stocks of the coronaviruses in high bio-safety labs. Once the desired quantities are produced, they are doused with a chemical called “beta-propiolactone”.
What does beta-propiolactone do?
The compound disables the coronaviruses by bonding to their genes. Therefore, inactivated coronaviruses could no longer replicate. But their proteins, including spike, are still whole — as in intact — which is useful in prompting response from our body’s immune response (antibodies, T cells and B cells).
BETA-PROPIOLACTONE
Beta-propiolactone, an organic compound of the lactone family, is used as inactivating reagent for vaccines. It is also used in tissue grafts, surgical instruments, and enzymes, as a sterilant of blood plasma and as a vapour-phase disinfectant.
What happens next?
The lab technicians scoop the killed viruses out and mix them with a tiny amount of an aluminium-based compound — called an “adjuvant” (adjuvants are normally stimulate the immune system to boost its response to a vaccine).
What is the efficacy rate of the Sinopharm shot?
The UAE, among the first to approve the Sinopharm shot, said the vaccine was 86% effective, based on interim results of Phase 3 trials in the Emirates.
Is it safe?
Yes. Millions have already received the vaccines in the UAE 5.5 million, most of them were Sinopharm shots, with no reports of severe reactions or adverse effects. Chinese media reported on February 20, 2021 that 43 million doses of Sinopharm COVID-19 vaccine had been administrated worldwide, of which 34 million were in China.
What did peer-reviewed journals say about earlier trials of the Sinopharm vaccine?
On August 13, 2020, JAMA published the interim results of two randomised Phase 2 clinical trials of the Sinopharm vaccine, and wrote: “This inactivated COVID-19 vaccine had a low rate of adverse reactions and demonstrated immunogenicity.” Following trial review and the results’ publication, the Chinese approved the vaccine, the first formal green light for a COVID-19 vaccine in the world.
2 BILLION DOSES FROM CHINA
The mainland currently has 18 production lines for COVID-19 vaccines with capacity for 2 billion doses by the end of 2021, and 4 billion by 2022, Feng Duojia, president of the China Vaccine Industry Association, told China Central TV on February 20.
What is the key advantage of the inactivated platform?
Less developed countries with no access to deep-freezing facilities would benefit from this type of vaccine. They can be transported more easily and at normal fridge temperatures. By comparison, Moderna and Pfizer’s vaccines, must be kept very cold — -20 °C to -70 °C. This poses a major logistical challenge, especially in places where power is unreliable or non-existent. Immunisation programmes even in developed countries, like the UK and the US, had been hampered because of the freezing requirement.
Does it offer 100% protection from moderate and severe forms of COVID-19? Yes, according to Abu Dhabi-based chief research officer Dr Walid Abbas Zaher, vaccine project leader of G42 Healthcare. It has an overall efficacy rate is 86%, he added.
“Think about the vaccine the same way you are taking the flu vaccine. If you are taking the flu vaccine, sometimes you don’t get the flu at all and sometimes you get the flu but in a very mild form. COVID-19 at the end of the day is a flu, a very nasty bad flu.
“And the vaccine works in the same way. It will either prevent you completely from having the disease or there is a remote chance that you get an infection. Even if you get it (infection) after taking the vaccine, you will not feel it. Even if you feel it, it will be a very mild flu.
EXTENSIVE PHASE III TRIALS IN THE UAE
The UAE Phase III trials revealed an 86% efficacy rate, a 99% eroconversion rate of neutralising antibody and 100% effectiveness in preventing moderate and severe cases of COVID-19.
Dr Nawal Al Kaabi, Chairperson, National COVID-19 Clinical Management Committee, said: “This is a safe vaccine. All the side effects that we have seen were expected and we have seen it with other vaccines — and maybe even less compared with other vaccines.” “The efficacy is really good. It has 100 per cent efficacy in preventing severe disease, which is great. What else would you want from a vaccine?” Dr Nawal, the Principal Investigator of Phase III Clinical Trial, asked.
SEROCONVERSION
In immunology, seroconversion is the period (usually measured in number of days) during which a specific antibody develops and becomes detectable in the blood, taken through a blood sample. After seroconversion has occurred, the antibodies can be detected in blood tests for the disease.
Does the Sinopharm vaccination mean 100% protection against infection?
No. Dr Nawal Al Kaabi explained: “(Though) you are protected 100 per cent against severe infection, there is a chance to acquire the disease. So, it is important for people who have taken the vaccine to continue to wear masks… and follow all the precautions.”
But the vaccines were developed too fast. How can we be sure no shortcuts were made, and that they’re really safe?
The question of safety is a scientific one, and the answer must be based on scientific evidence, not scaremongering posts or memes on Facebook by alarmist vloggers. It’s true that speed of vaccine development for SARS-CoV-2 has been unprecedented. But the involvement of numerous governments, including funding support, for vaccine development has been unprecedented too.
With money not an issue in launching several costly trials, at least 8 vaccines were approved within 12 months from the starting point of human trials. It used to take at least 4 years and billions of dollars of private money to develop drugs and vaccines (that’s why they’re expensive, too).
Can the length of vaccine development guarantee its efficacy and safety?
No. The length of development of a vaccine or drug but is not a guarantor for its safety and efficacy. Some, like the HIV vaccine, has been under development for more than 20 years, but many trials showed no efficacy.
How long will protection last? What about reinfection?
Nature wrote that the durability of protective immune responses remains “unknown”: “Although the preponderance of evidence suggests that adaptive immune responses elicited by SARS-CoV-2 infection are present and might protect against re-infection, experience with seasonal coronaviruses and present experience with SARS-CoV-2 suggest that immunity to natural infection might wane over time, and reinfection has been reported.”
I had been diagnosed with COVID-19 in the past. Should I get this vaccine?
There are people previously infected a COVID-19 infection who had taken the vaccine, with no issues. Nature said it remains unclear whether previously infected persons would benefit from vaccination. However, it the journal states vaccination against SARS-CoV-2 should be made irrespective of infection status (whether someone had been infected or not).
What about the quality of every batch of this vaccine produced?
This is actually a good question. Quality is of utmost importance. That’s why strict inspections and quality controls every step of the biosafe environment and manufacturing processes must be adhered to. This is because both safety and efficacy can be affected by even minor lapses in quality.
How is vaccine quality assured?
Random and regular inspection of every part of the manufacturing process and every batch of vaccine produced also form part of quality controls. There are country-specific regulatotions as well as international standards developed by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH), which forms the basis for ensuring quality in clinical research.
The “ICH Q” guidance series, on the other hand, ensures that vaccine quality meets global standards for bio-chemistry, manufacturing and control. It’s known as “GxPs” (that is, Good Clinical Practice, Good Manufacturing Practice and Good Laboratory Practice). Compliance ensures the vaccines being deployed have consistent safety and efficacy. That’s critical to overcome vaccine hesitancy.
How many other COVID-19 vaccines have been developed in China?
Besides CNBG’s two vaccines, China’s emergency-use programme also includes a shot from Sinovac Biotech. A fourth candidate from CanSino Biologics jointly developed by the Hong Kong-listed CanSino and the Beijing Institute of Biotechnology (part of China’s Academy of Military Medical Sciences), was approved for use in for Chinese military personnel in June 2020.
Is it possible to have a smaller dosage of the vaccine, but with the same efficacy?
Smaller, or fractional dosing, has been discussed by experts and vaccine developers as a possible measure to fight COVID-19. However, exploring “dose optimisation” takes time. Obviously, the vaccine trial schedule has been greatly reduced by the urgency of the need to develop them as soon as possible. But as vaccinations cover more areas of the world, more data will emerge.
Historically, Nature pointed out, fractional dosing has already been used to extend the supply of yellow fever vaccine. If lower doses of COVID-19 vaccines prove efficacious and safe, then, particularly when supplies are limited, fractional doses might allow more people to be vaccinated. Janssen’s one-shot vaccine regimen is seen as a game changer in the fight against the SARS-CoV-2.
There’s another: a study published in the Lancet shows Pfizer’s first shot itself guarantees an up to 85% immunity against COVID-19 after 2-4 weeks. The survey was done on 9,000 health worker in Sheba, Israel’s largest hospital (about 7,000 received the first dose, while the rest were not inoculated).
Will experts, given the new data, then recommend just a single Pfizer shot, instead of the current two? It remains to be seen. If the answer is in the affirmative, this theorically could double coverage with current and future production. If so, it would greatly bump up the multi-pronged fight against the pandemic.